With summer in full swing (an understatement in much of the country), everyone is talking about sunscreen, as new research emerges putting into question the safety of a whole host of common sunscreen ingredients, including nano zinc oxide. And with good reason.
The rise in prevalence of nano sized particles across a wide range of products has been largely absent any sound research demonstrating their safety. Moreover, it’s largely accepted that these teeny weeny microscopic particles are small enough to penetrate the skin and enter the bloodstream, which begs the question: To what extent do nano particles get into the body, and what do they do when they get there?
The answer to that question is, with regard to most nano particles, an unsatisfying, “We don’t know.”
One exception, however, is nano zinc oxide. According to Bob Root, Keys Care‘s lead formulator and the genius behind top-rated Solar Rx, there are over 400 published studies on the behavior of nano zinc oxide when used in creams and lotions (far less data exists on powders and sprays – i.e., the kind you can inhale). In fact, Root states the bigger problem with nano zinc oxide is making it adhere to the skin. “The spherical shape of nano zinc causes the skin to push it back out,” he explains. “Other nano particles that have more rugged shapes seem to penetrate better, but with nano zinc, the challenge is keeping it on the skin.”
 Considering Root and his wife, Wendy Steele, created Keys Care in response to Steele’s battle with melanoma, it is no surprise they are passionate (and extraordinarily knowledgeable) about sun protection. It is because of his determination to create the safest and most effective sunscreens that Root uses nano zinc oxide; he firmly believes it does a much better job protecting skin from the sun’s dangerous UVA rays. His rationale is pretty compelling; to explain the difference, he uses the anology of rocks vs. sand.
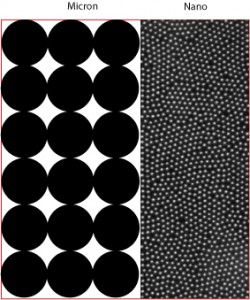
Considering Root and his wife, Wendy Steele, created Keys Care in response to Steele’s battle with melanoma, it is no surprise they are passionate (and extraordinarily knowledgeable) about sun protection. It is because of his determination to create the safest and most effective sunscreens that Root uses nano zinc oxide; he firmly believes it does a much better job protecting skin from the sun’s dangerous UVA rays. His rationale is pretty compelling; to explain the difference, he uses the anology of rocks vs. sand.
“If you spread rocks out on one piece of red carpet, and sand on another, on which piece would you see the most red?” In other words, not only does nano zinc fail to penetrate the skin and get into the blood stream, but the smaller particle size means fewer “holes” when you apply a layer of nano zinc oxide sunscreen on the skin (see his illustration on the left).
The Environmental Working Group would appear to concur with Root’s assessment, giving the green light in their 2011 Sunscreen Report to numerous sunscreens that use nano zinc oxide as their primary source of UVA and UVB protection. While the jury is still out on the environmental impact of these itty bitty minerals, it would seem we can all rest easy when it comes to breaking open a bottle of Keys Solar Rx, Vive Sana, Blue Lizard, or Soleil Organique. All use nano zinc oxide to provide excellent broad spectrum protection, and all made the EWG’s Best Sunscreens List of 2011.
Still not convinced? Well, good news: You can still find plenty of excellent sunscreens that claim not to use nano zinc oxide. Eco Logical and All Terrain (available at many local co-ops and health food stores) are two of my favorites, and neither leaves any hint of white when applied to all but the darkest skin tones. I would add, however, that Root firmly believes if the sunscreen goes on clear, no matter what the manufacturer claims, you are using nano sized zinc oxide and/or titanium dioxide.
If you would like to learn more, Root wrote an excellent, in-depth piece on the Keys Care Blog in 2009 on the topic of nano zinc oxide. You can also hear my entire interview him here, or download it on iTunes. Finally, be sure to check out the EWG’s 2011 Sunscreen Report if you haven’t already, for a list of the best sunscreens, worst sunscreens, and the latest information on sun protection and ingredient safety.




3 Comments